- a wine glass, preferably with an almost spherical bulb, such as the one use for Rhine wine
- about 100 ml of water, coke, or coffee; note: we need a balance to measure the mass of the water; or use a cooking measure to determine the volume
- digital thermometre; it should give readings of 0.1 degrees.
- clock or watch
- piece of cardboard or wood, large enough to completely shield the wine glass from direct sunlight and to provide a good shadow

The dark liquid will absorb the sunlight and convert this energy into heat, so we can measure this by observing how fast the glass heats up under sunshine. However, the liquid will also lose some of its heat by conduction through the glass and simply by radiating itself in the infrared. We can determine this heat loss by observing how fast the liquid cools down, once we put the glass in a shadow, and thus is has no longer any power input from the sun.
Experimental procedure
- fill the wine glass with a carefully measured quantity of water, coke, or coffee. If you have plain water, add some instant coffee until the colour is really dark
- put the glass near the window, where it would be in full sunlight, but carefully shield it from direct sunlight, and wait until the temperature of the liquid has reached a constant value. Record this temperature, which is the ambient temperature.
- now remove the shield, and measure the temperature every 30 seconds (or any interval to capture nicely the rise of the temperature), while using the thermometer's sensor to gently stirr the liquid. Record your measurements.
- continue this until the temperature becomes a constant value
- then put the sun shield back in place, or move the wine glass into a shadow nearby. Continue to take temperature measurements, until the temperature reaches again a constant value, which will be close to the ambient temperature we had measured in the beginning. Since the cooling will take a longer time, the intervals for the measurements can be longer, but we also have to wait longer!
Interpretation
The dark liquid is nothing but a body which heats up as a result of power input. However, this body is thermally not perfectly isolated, so it loses some of its heat. It is very reasonable to assume that this loss rate is proportional to the difference between the temperature T of the body and the ambient temperature T_a, we can describe the time variation of the body's temperature T by this equation
The left hand side is the rate of change of the heat necessary to cause the temperature change, the right hand side consists of the heating term and the heat loss rate. Here, c is the specific heat of the body (water = 1 cal/g/deg), m is the body's mass in grams, P(t) is the power absorbed at that moment, and a is the coefficient for the heat loss. If the input power is constant, this equation has the solution
which tells how the body's temperature changes with time, from its initial temperature T(0).
There are two ways for the analysis: The first one employs the fact that we may use directly the rate dT/dt of the temperature increase measured right at the beginning, when the body's temperature was close to the ambient temperature, and so we can still neglect the heat loss
The mass m and the specific heat of the body are known. The power absorbed by the body
is the product of the cross section A (in cm²) of the body and the "Solar Constant" (= 500 W/m² at the Earth surface). The factor 4190 converts the power unit W into calories that are easier to work with, while the factor 60 enters because it is more convenient to use minutes as a time unit! Putting things together, we arrive at these formulae
which gives the absorbed power in W. For the solar constant we get
in W/m².
The above simple formulae depend on one single, but critical measurement: dT/dt or how fast the temperature rises in the beginning! The second method uses more data points and is hence more reliable. It runs in two steps: the first part is the heating up under the effect of the constant sunlight:
Here the power absorbed is as above
We also know the ambient and initial temperatures T_a = T(0) from our reading before the start of the heating up.
However, we do not know yet the loss coefficient a! For this purpose, we put the wine glass in the shadow and let it cool off. As there is no longer any power input, the temperature would follow this formula
where T(max) is the temperature at the start of the cooling off experiment, hence the maximum temperature after heating up.
We could use the last equation to deduce the constant a and the temperature T(t) measured at some time t while the cooling was still progessing:
But a better approach is to plot the measurements together with the curve of the time evolution of T(t) predicted from the formula above for a guess value for a, and playing with that value until a best fit of all the measurements are obtained, such as seen below
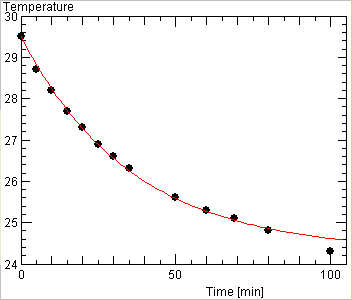
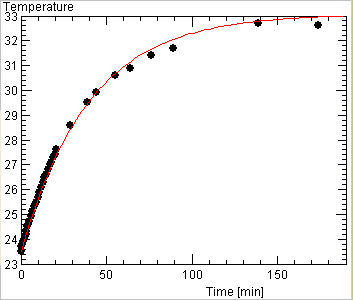
An example of such a fit is shown below
Further experiments
Do the experiment with clear water instead of dark coffee: you will find that the power is less. Clear water does not absorb solar radiation as easily as a dark liquid.
Do the experiment behind the glass pane of the window: you will also find that the absorbed power is less than when the window is open. Glass does absorb a certain fraction, in particular the ultraviolet light. Doing these experiments allows to measure how much the glass absorbs.
When we do the experiment in the open window, we have to be careful: Any wind passing by the glass will cool it, and so it might appear that with the open window we get a lower value for the absorbed power. So we may have to protect the glass against movements of air by placing some objects such as books to act as screens. But we have to be careful that these objects do not reflect sunlight to to glass ...
We also have to be careful when there are clouds in the sky. When a cloud passes over the Sun, it diminishes the light falling on the glass, and thus we will obtain a value too low.
The same experiment can be done to measure the light output of a lamp. The example curves above were obtained in this way and showed that the efficiency of a normal desk lamp bulb is only 3 percent - but that includes the infrared emission which is useless for lighting!

Instead of a glass of water we may use any body whose temperature we can measure. An example is a small block of blackend aluminum - what was a heat sink for an electronic device. We need to weigh its mass, and we also know the specific heat of the material 0.2 cal/g/deg. A lower specific heat means that one needs less heat to raise the temperature by the same amount. Hence this block of metal gets fairly hot under the sun, and it heats up rather quickly ... but it works in the same way!
The data from my experiments can be found and analyzed with this JAVA applet
| Top of the Page | Back to the MainPage | to my HomePage |
last update: March 2010 J.Köppen