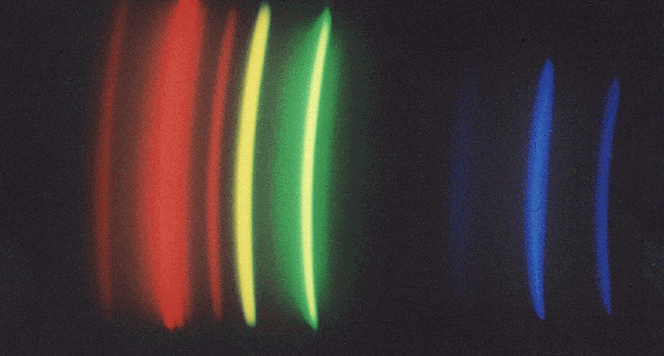How to see the spectra of street lamps
Joachim Köppen Kiel/Strasbourg/Illkirch Spring 2007
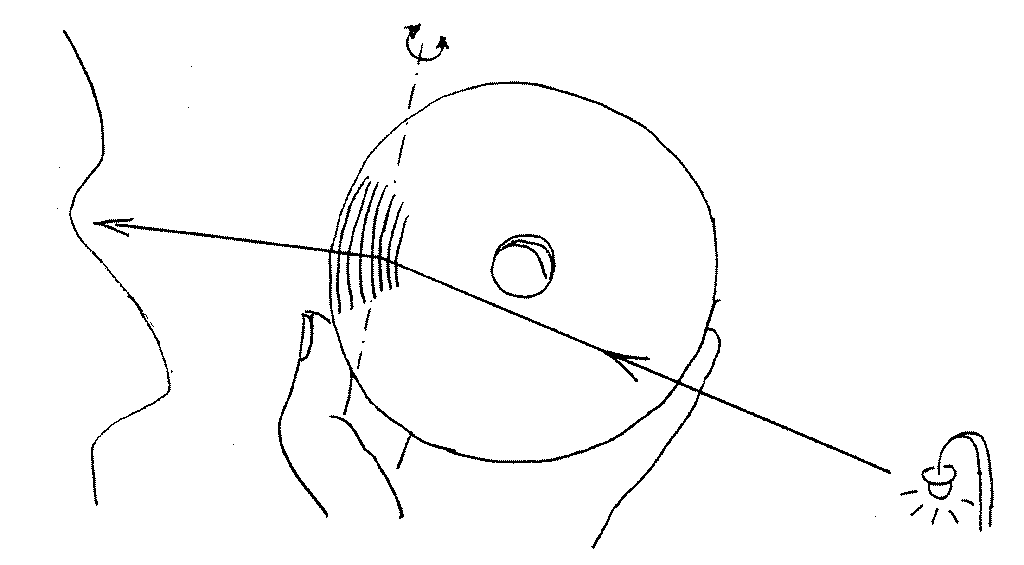
The darkness of the evening or night offers the easiest way to observe
spectra with a CD, when we look at the reflection in a CD of a distant
bright small source, such as a street lamp or a bright window. The
procedure is this:
- hold the CD so that you get the directly reflected image
of the source. You may turn your back to the lamp and
view the CD turned towards it, or you may hold the CD
directly in front of your eye and turn it 45 degrees as to
look sideways (as in the above sketch). The exact position
does not really matter.
- when you have the direct image in one side of the disk, swivel
the CD by about 10° towards one side until a coloured band
appears or a few bright arcs, each of a different colour.
Violet will be on the side closest to the direct image of the lamp.
If you follow the above sketch, you should turn the CD towards
the left.
- It may be that you see only a series of bright dots ...
then you should turn the CD back to the direct image, and
keep turning until the band or arcs appear.
Since the tracks on the CD are curved, the spectrum on one side
is broad (in vertical direction) but narrow in the other side
of the direct image.
- if you turn the CD a bit further, a second
spectrum is seen. This second-order spectrum is not as bright
as the first one, but its colours are more strongly spread out.
There is even a third spectrum which partially overlaps with
the second one.
- look at the first order spectrum, and change the distance
of the CD from your eye. At about 15 cm the spectrum will have
spread (vertically) covering the whole disk, as each colour
is spread out over a large arc. Rather spectacular!
The spectrum tells you about the nature of the light source:
if you see a continuous rainbow-coloured band with all
colours being equally bright, the spectrum is continuous and comes
from the surface of a hot solid body such as the tungsten wire
in an incandescent lamp. Also, high-pressure metal vapour lamps
have continuous spectra. But if only bright spots or arcs are seen,
the spectrum contains emission-lines, and the lamp is most likely
of a low-pressure metallic vapour type. For instance,
mercury vapour lamps show only a red, an orange (589 nm from sodium),
a yellow (578 nm), a green (546 nm) and a violet line (at 436 nm;
young people may see the other violet line at 405 nm);
sometimes there is a bit of continuous emission in the blue.
One example is shown here:
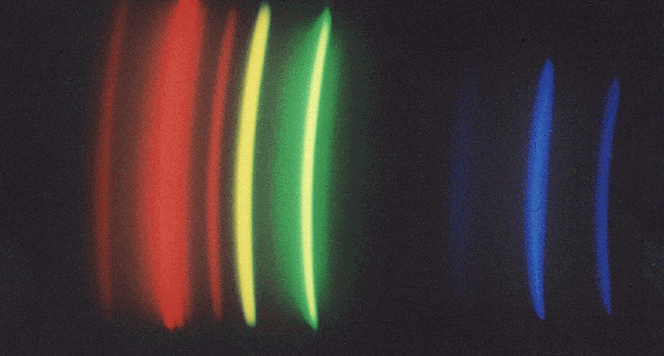
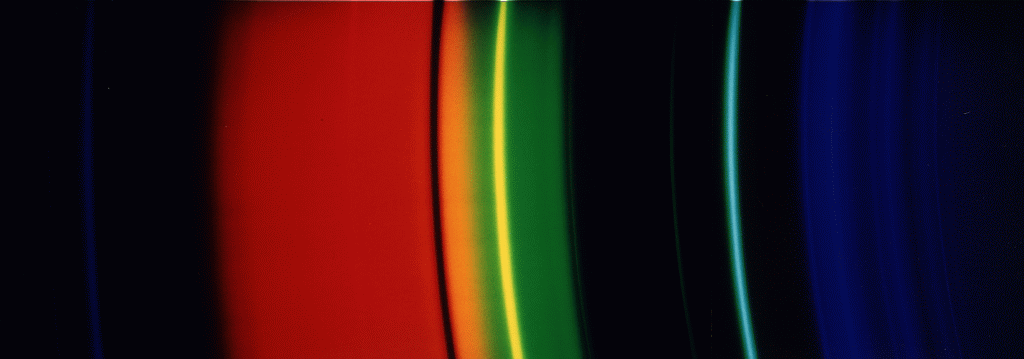
A sodium-vapour lamp gives off the orange line you also see in
the solar spectrum. However, the lamp in front of my appartment
- like most sodium lamps - shows a dark gap where one should
expect the orange line. The line is much more intense on its 'wings'
than in its centre ... Though the main emission is in the orange, there
are a number of other lines, too. Also, sometimes, one finds weak lines
from mercury, which is also used in the sodium lamps.
| Top of the Page
| to Main Page
| to my Home Page
|