The spectrum of the Sun
Joachim Köppen Kiel/Strasbourg/Illkirch Spring 2007
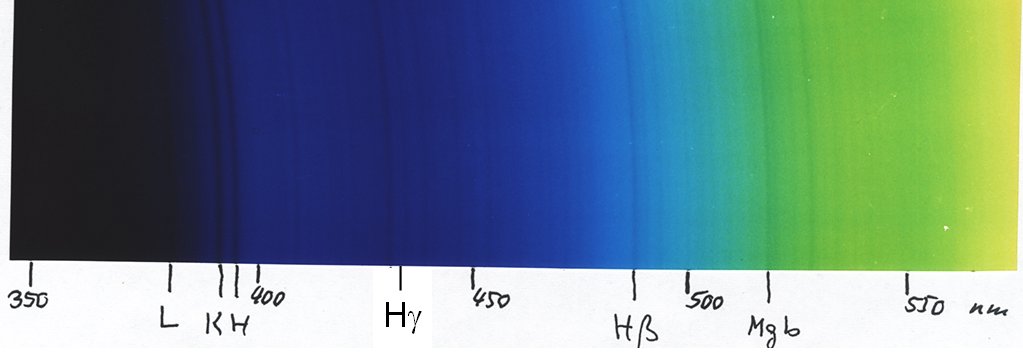
Here is the solar spectrum, done with the Mk III version starting with the violet of the second order:

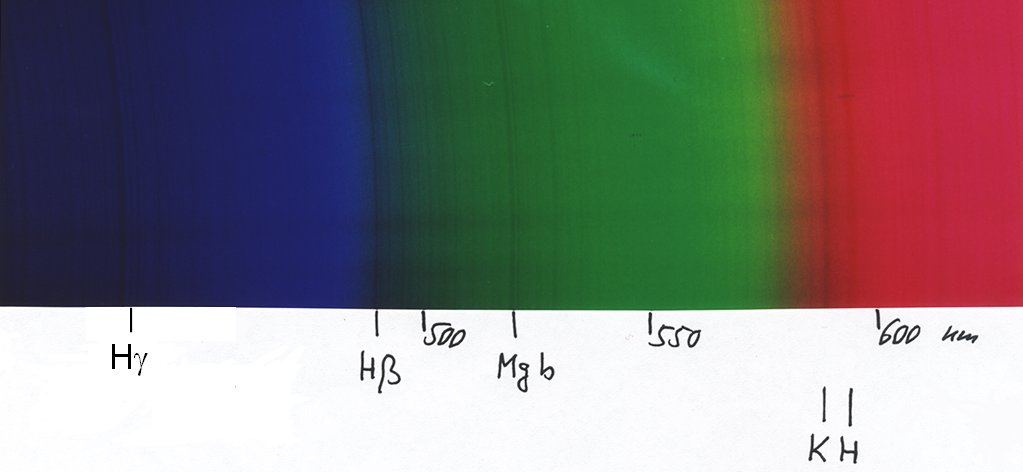
... here we continue into the green and red. Note that the yellow colour does not show up, because it is represented in the fiml by the overlap of red and green, but apparently the intensities were not high enough for the film prints to give a bright yellow ...
... the red second order spectrum overlaps with the violet part of third order, thus giving the pinkish colour ...
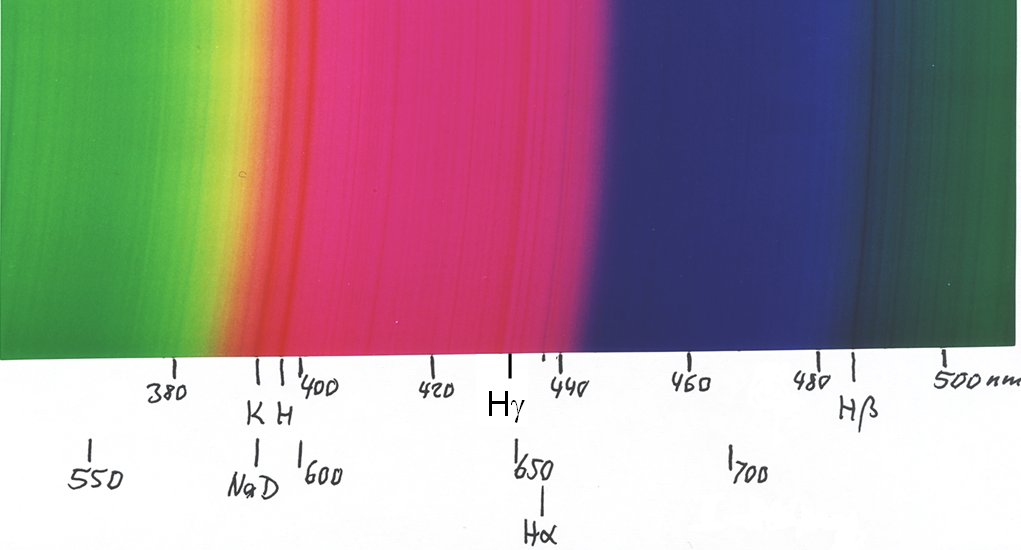
... and this is the green third order, which already overlaps with the extreme violet of fourth order, as seen by the H and K lines:
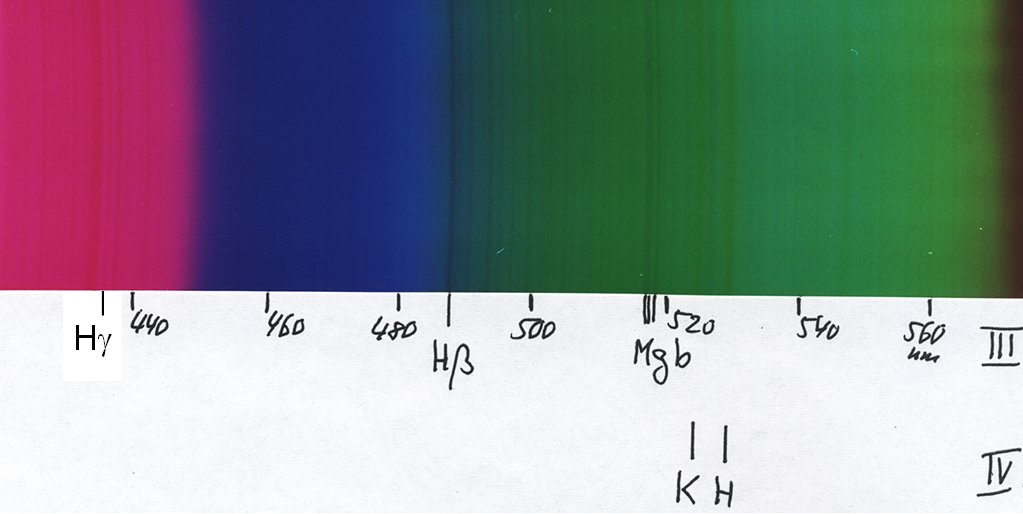
Below is a table of the dark lines in the solar spectrum. The first column gives the traditional designation of a line (given by Fraunhofer), the second is the wavelength in Ångström (5000 Å = 500 nm), the third gives the element or ion responsible for the line, and the last column gives the "equivalent width" in mÅ ( = 0.001 Å), a measure of its darkness. Spectral lines are not completely black, but they show a smooth profile of the intensity going from full intensity down to some value. Therefore one measures the strength of a line by giving the width of a completely black region which would absorb as much light as the line.
Note that with our eyes we cannot easily see light with wavelengths shorter than about 4300 Å, so the very strong H and K lines of ionized calcium are invisible for us - but young persons may be able to do that! Luckily, photographic emulsions go a bit further. For example, with a normal colour negative film, I could easily photograph the mercury line at 4050 Å.
| Designation | wavelength (Å) | Identification | eqivalent width (mÅ) |
| M | 3734.87 | Fe | 3027 |
| L | 3820.44 | Fe | 1712 |
| K | 3933.68 | Ca+ | 20253 |
| H | 3968.49 | Ca+ | 15467 |
| Hd | 4101.75 | H | 3133 |
| g | 4226.74 | Ca | 1476 |
| G', g | 4340.48 | H | 2855 |
| d | 4383.56 | Fe | 1008 |
| F, b | 4861.34 | H | 3680 |
| b4 | 5167.33 | Mg | 935 |
| b2 | 5172.70 | Mg | 1259 |
| b1 | 5183.62 | Mg | 1584 |
| E | 5269.55 | Fe | 478 |
| D2 | 5889.97 | Na | 752 |
| D1 | 5895.94 | Na | 564 |
| C, a | 6562.81 | H | 4020 |
| B | 6867 | O2 (Earth atmosph.) | -- |
| A | 7594 | O2 (Earth atmosph.) | -- |
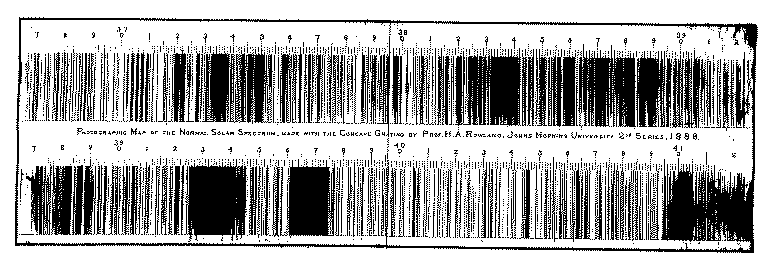
And this is a small part (from 3670 to 4120 Å) of the solar spectrum by H.A.Rowland, obtained more than 100 years ago: